新帕泰克公司执行一整套完整的规范的研发程序来对产品进行设计开发和生产,该程序业经医药行业内的众多公司的严格审核和验证。该整套程序详细记入 标准执行程序 (SOPs).
所有对新帕泰克公司仪器、软件、程序、文件等的改进意见(来自用户、销售人员、服务人员、生产人员等),可以通过任何渠道反应给我们,按接收到的时间顺序归类,储存入信息库,并列入“改进建议表”
定期摘录这些建议和要求,并把摘录内容列入发展规划项目中。
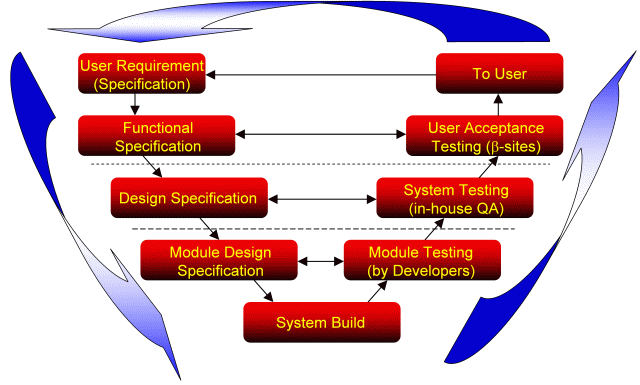 新帕泰克对各种开发项目基本采用上图所示的"V型"为标准循环检测周期模型,
新帕泰克对各种开发项目基本采用上图所示的"V型"为标准循环检测周期模型,
注:这种方法不仅限于软件开发
All development work writing a functional specification in the language of the "user". Simultaneously the criteria for the user acceptance test are defined in the same document: Each request has a direct relation to the related test. So at this level the "user" knows exactly what he will get at the end of the projects.对所有的开发工作,首先是功能要求,使用人员是“用户”,用户验收文件的标准也相应的定义入同一个文件中。每个要求都同相关试验有直接的联系。在此层面上的“用户”能够非常清楚的知道最终他能够得到什么。.
In a second step the functional specification is converted into the design specification which is know written in the language of the developer. This happens simultaneously with the user acceptance test, which is converted into the system test. Again, each request has a direct relation to the related test. So at this level the "developer" knows exactly what he will get at the end of the project第二步,功能要求转化为设计规范,此层面的人员是开发者,用户验收文件相应的转变为系统验收文件,同样,每个要求都同相关试验有直接的联系。开发者能够非常清楚的知道最终他能够得到什么。
For software developments often a third level, the module design specification and the corresponding module testing are derived from the previous level, allowing to split larger tasks into small modules
对软件的开发通常处于第三层次,模块设计要求和模块功能试验和前面的层次相关的,这样就把非常大的任务程序分割成小的独立的模块。.
The entries of all levels are connected traceable by the request and test numbers在各个水平层面上的主体数可以直接上溯到要求和试验的数目。
Finally a Gantt diagram assigns resources and time budgets to the individual tasks forming the overall project schedule最后,一张Gantt图将不同的步骤和相应的预算时间综合起来,得到整个开发项目的详细的日程进度安排。.
Now the project work can start and the results are tested after completion using the pre-defined test criteria of the three levels. So the user gets exactly what was defined in the beginning at the user level于是,项目开始了,不同层次的人员根据不同的试验规范得到不同的结果。用户可以得到其在最开始的时候设定的要求。
| 备注: | 一个明确的开发过程,采用循环检测周期模型的先决条件,是开发的软件符合 21 CFR 规则11. |
|
基本信息 |
| SOP's 标准操作程序 |
| 循环检测周期模式 |
| 参考 样品 |
|
品质 保证 |
| 21 CFR Rule 11 |
|
技术支持 |
| 评估确认 |
|
信息 |
| 技术文章 |
| ISO 标准 |